 NEUROPSYCHOLOGY OF ENDOCRINE DISORDERS
NEUROPSYCHOLOGY OF ENDOCRINE DISORDERS
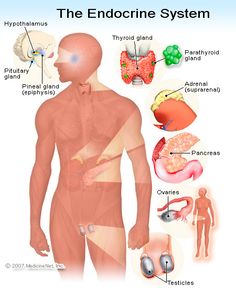
The endocrine system (ES) is composed of a diverse array of components whose functions are equally as diverse, both in terms of function and dysfunction. The relationship between the ES and central nervous system (CNS) is comprised of multiple feedback loops (Hadley & Levine, 2006; Blumenfeld, 2002) so that disorders of the ES may initially present with neurological deficits, while neurological deficits may be exacerbated by concomitant ES dysfunction (Goetz, 2003). As such, developing an understanding of the ES and its components and the hormones involved is essential when working with children and adolescents, particularly within the context of dysfunction.
The purpose of this section is to introduce the reader to the anatomical and chemical substrates of the ES, their importance for the developmental process and the consequences when the system malfunctions.
Disorders of Thyroid Function
The thyroid gland is located in the front of the neck and typically weighs around 30 grams. Its role is to synthesize and secrete two essential hormones: triiodothyronine (T3) and tetraiodothyronine or thyroxine, as it is more readily known (T4). Both hormones are synthesized from tyrosine (Hadley & Levine, 2006). T3 and T4 are involved in the regulation of tissue metabolism. Production of T3 and T4 in the thyroid is regulated by the pituitary gland, specifically through secretion of thyroid stimulating hormone (TSH), whose synthesis is regulated by hypothalamic secretion of thyrotropin-releasing hormone (TRH) (Straight, Bauer, & Ferry, 2003). There is a negative feedback loop wherein serum T4 regulates the release of TSH and TRH. In general, thyroid hormones are involved in the development of the CNS, maintenance of muscle mass, control of skeletal growth and differentiation and the metabolism of carbohydrates, lipids and vitamins (Porterfield & Hendrich, 1993; Zoeller, Bansal, & Parris, 2002). In their absence, physical and mental development is impaired.
Disorders of the thyroid gland can be divided into those that involve either an under production of hormones, (i.e., hypothyroidism), and those that involve an overproduction of hormones, (i.e., hyperthyroidism), each with distinct impact on physiological and, therefore, cognitive and behavioral functioning.
Hypothyroidism
Hypothyroidism is the most common disorder of the thyroid gland in both children and adults and is twice as common in females as in males. It can be caused by dysfunction of the thyroid gland itself (primary hypothyroidism), a disturbance outside of the thyroid involving the pituitary or hypothalamus (central hypothyroidism), or resistance to thyroid hormone (Goetz, 2003). The prevalence of congenital forms of hypothyroidism (e.g., due to abnormal development or location/position of the organ) is 1 case per 3,500 in the population. Secondary and tertiary forms are rare (1 per 60,000 and 1 per 140,000 newborns worldwide respectively; Straight, 2003). The development of thyroid dysfunction can result from destruction via viral or autoimmune damage, chronic inflammation with lymphocytic infiltration, cell death resulting from radiation damage, or defective hormone production. If left untreated in the newborn, hypothyroidism leads to profound growth failure and disrupted CNS development, the latter resulting in a condition called cretinism (Rovet & Hepworth, 2001). The age of onset of symptoms in children is unpredictable because initial increases in TSH may help compensate for insufficiency due to congenital malformations of the thyroid gland. Primary hypothyroidism is the most common form of hypothyroidism and most commonly affects women between the ages of 40 and 60 years (Goetz, 2003). Dysfunction of the hypothalamus or pituitary contributes to hypothyroidism in only 10 percent of cases.
Amongst the earliest signs of congenital hypothyroidism (CH) in the infant are prolonged gestation, increased birth weight, constipation, prolonged jaundice, poor feeding and management of secretions, hypothermia, decreased activity level, noisy respirations and a hoarse cry (Postellon, Bourgeois, & Varma, 2006). The clinical features of the acquired form of hypothyroidism include goiter (i.e., a thyroid that appears “swollen” or “full” resulting in complaints of difficulty swallowing, hoarseness or feeling of pressure on the neck), slow growth with delayed bone maturation, mild increased weight in the torso area despite decreased appetite (moderate to severe weight gain/obesity is not characteristic of thyroid dysfunction and suggests the presence of other issues), lethargy and decreased energy, dry skin and puffiness, sleep disturbance (most typically obstructive sleep apnea), cold and heat intolerance, weight loss, tremors, and sexual pseudoprecocity (e.g., testicular enlargement in boys and early breast development and vaginal bleeding in girls; Straight, 2003).
Intellectual functioning in children with hypothyroidism has been an area of significant interest. In a review of the research literature, Derksen-Lubsen and Verkerk (1996) determined that there was a trend towards lower intelligence quotients (IQ) in children who were diagnosed and treated for congenital hypothyroidism. In fact, these children as a whole had IQ levels that were approximately 6 points below that of control participants.
Not all studies have demonstrated decrements in intellectual functioning between hypothyroid and euthyroid children (i.e., those with normal levels of circulating thyroid hormones). For example, Bongers-Schokking and De Muinck Keizer-Schrama (2005) found fact there were no differences in global IQ scores for children between the ages of 5 years, 6 months to 7 years old. However, they found that IQ correlated significantly with the level of treatment. For instance, children who received high levels of levothyroxine, a medication used in the treatment of hypothyroidism, tended to have higher IQ scores in comparison to children who received a lower level of the medication. Moreover, the children receiving high levels of levothyroxine had higher IQ scores even relative to control participants. Children who were treated with low or suboptimal levels of levothyroxine tended to have the lowest levels of IQ scores albeit remaining in the average range.
Some researchers have attempted to clarify the issue of IQ and hypothyroidism by stratifying groups based on level of severity of the condition. Kempers and colleagues (2006) evaluated intellectual and motor development of young adults who were diagnosed with congenital hypothyroidism during the neonatal period. The patients were tested initially at 9.5 years of age and again at 21.5 years of age. The patients were divided into mild, moderate and severe congenital hypothyroidism groups based on laboratory assays of T4. Intelligence scores did not differ significantly from testing at time one to time two regardless of severity of CH; however, the severe CH group had significantly lower Full Scale, Verbal and Performance IQ scores during each of the testing periods in comparison to those patients with moderate or mild levels of hypothyroidism. Results for the mild and moderate groups were fully within the average range while results for the severe CH patients ranged from average to low average.
Recently, research has begun to focus on children who were diagnosed with compensated or subclinical hypothyroidism. This group of children tends to fall towards the lower end of the normal range for levels of free T4, and TSH is typically normal as well. Aijaz and colleagues (2006) found that this group of children did not differ from control participants in terms of verbal and nonverbal reasoning, suggesting that the severity of hypothyroidism, as with the work by Kempers and colleagues (2006), is likely the determining factor in level of intelligence.
Research on the neurocognitive functioning of individuals with primary hypothyroidism indicates multiple areas of deficit in addition to intelligence. Wekking and colleagues (2005), in a study of adults treated for congenital hypothyroidism in the , found deficits in complex attention using the Paced Auditory Serial Addition Task (PASAT; Aarnoudse, van den Burg, & Saan, 1995 ). The participants had greater difficulty with single trial learning relative to the reference group on a measure of verbal memory (Rivermead Story Recall; Wilson, Cockburn, & Baddeley, 1985), but performed on par or better than the reference group when multiple practice trials were involved (California Verbal Learning Test (CVLT; Delis, Kramer, Kaplan, & Ober, 1987 ). Using the Symptom Checklist 90 (SLC-90; Derogatis & Savits, 1999), Wekking and colleagues noted that their adult sample reported lower psychological well-being than the reference sample, including greater levels of depression, nervousness and fatigue.
Pediatric research has noted a similar trend in terms of neurocognitive deficits. In their review of the research on patients who were treated early in development for congenital hypothyroidism, Derksen-Lubsen and Verkerk (1996) reported a trend towards lower scores on measures of intelligence and motor skills. Kempers and colleagues (2006), in addition to the lower IQ scores relative to matched controls, found that individuals diagnosed with CH via neonatal screening also evidenced motor deficits including manual dexterity, ball skill and balance and specifically noted that the deficits evidenced during childhood did not improve by the time the participants reached young adulthood. Zoeller and Rovet (2004) reviewed the clinical and experimental findings of the timing of thyroid action on brain development. They concluded that it is the level of thyroid hormone that is present in the fetus and neonatal system that determines the type of deficits that may be encountered. In general, delaying treatment was found to have a greater impact on visuomotor and language skill development, indicating that these areas of cognitive functioning are particularly sensitive to the level of thyroid hormone in the child’s system. Summarizing across the various studies that were reviewed, Zoeller and colleagues concluded that prenatal thyroid hormone loss contributes to deficits in visual processing and motor and visuomotor abilities. Deficiencies occurring during the early neonatal period are associated with impaired visuospatial abilities, while insufficiency that occurs somewhat later during postnatal development is associated with sensorimotor and language impairments. Even later during infancy, thyroid hormone deficits are associated with language, fine motor, auditory processing, attention, and memory deficits.
Animal models have lent some support for the conclusions noted above. For example, Friedhoff and colleagues (2000) found that there was a gender difference in rats with prenatal hypothyroidism, in that females were more sensitive than males, resulting in learning problems. Furthermore, animals that were treated differed from those that were exposed to thyroid hormone insufficiency throughout the perinatal period. Animals that were treated tended to exhibit learning deficits and increased motor activity, while those that were exposed to chronic thyroid hormone insufficiency exhibited reduced motor activity. This finding suggests a link between the level of thyroid hormone and the development of symptoms often associated with Attention-Deficit/Hyperactivity Disorder (ADHD). In fact, Rovet and Hepworth (2001) found that adolescent participants with congenital hypothyroidism struggled on measures that required them to maintain their focus and inhibit inappropriate responses on a measure of continuous performance. More severe cases of congenital hypothyroidism were associated with additional difficulties including requiring more practice trials in order to encode verbal information, thereby affecting learning ability. Rovet and Hepworth also found that relative to the control participants, the clinical sample demonstrated a higher rate of commission errors (i.e., responding to the incorrect stimulus)on a measure of continuous performance and tended to work at a slower rate than controls on a measure of scanning speed. On the other hand, clinical participants were not significantly slower when required to maintain two sets of competing data in mind. Finally, the hypothyroid adolescents were less accurate and more prone to perseverative errors on the Wisconsin Card Sorting Test (WCST; Heaton, 1981), indicating decreased executive functioning.
Not all research points to deficits as a result of hypothyroidism. Wu and colleagues (2006) conducted a study in which they examined academic functioning in a group of children diagnosed with subclinical forms of hypo- and hyperthyroidism relative to euthymic controls. They found that the children with subclinical hypothyroidism actually performed better than the other two groups in terms of arithmetic and reading skills on a measure of academic achievement (Wide Range Achievement Test, Revised; Jastak & Wilkinson, 1984.) and also obtained higher scores on a measure of visuomotor integration (Block Design of the Wechsler Intelligence Scale for Children, Revised; Wechsler, 1974).
The presence of mental health-related issues in children and adults with hypothyroidism is undisputed, but the research is relatively scant, which points to the lack of sufficient interaction between mental health practitioners (psychologists and psychiatrists) and the field of endocrinology. The most common mental health-related issues that have been the target of research are associated with depression and anxiety. Goetz (2003) reported central nervous system features in individuals with hypothyroidism that included forgetfulness, inattention, apathy, and slowing of speech, movement and mentation, all of which are frequently associated with mood disorders (American Psychiatric Association, 2000). Jorde, Waterloo , Storhaug and colleagues (2006), in a study of 89 adult participants with subclinical hypothyroidism, found an association between lower levels of T4 and the presence of depressive symptoms, which tended to remit with institution of proper treatment. Similarly, Wekking and colleagues (2005) found that adults with congenital hypothyroidism tended to report lower levels of well-being relative to euthymic controls.
Storch and colleagues (2004) looked at the psychological correlates of peer victimization in children with endocrine disorders and found that acts such as bullying were positively related to child reports of depression, social anxiety, loneliness and parent reports of externalizing symptoms. Regarding psychological variables, the authors found that 7.5% of the children in their sample reported clinically significant depressive symptoms using the Children’s Depression Inventory (CDI; Kovacs, 1992), 5.7% reported clinically significant levels of loneliness on the Asher Loneliness Scale (Asher, Hymel, & Renshaw, 1984)and 19.8% of the youth reported elevated levels of social anxiety using the Social Anxiety Scale for Children-Revised (LaGreca & Stone, 1993).
Hashimoto’s Thyroiditis
Hashimoto’s Thyroiditis (HT) is part of the spectrum of autoimmune thyroid disorders (AITDs). It is characterized by the destruction of thyroid gland cells through the action of various cell- and antibody-mediated mechanisms (Odeke & Nagelberg, 2006) that are beyond the scope of this chapter. In HS, the thyroid is typically goitrous or atrophic (i.e., decreased in size), but may be normal in size. Patients often present with antibodies to various thyroid antigens, most commonly antithyroid peroxidase, antithyroglobulin, and, less frequently, with TSH receptor-blocking antibodies. There also is a small group of individuals who are antibody negative. Regardless of which mechanism or combination thereof the patient presents with, the result of HT is inadequate production and secretion of T3 and T4.
Both internationally and in the , HT remains the most common cause of hypothyroidism in individuals older than 6 years of age. In one epidemiologic study of children, Rallison and colleagues (cited in Radetti and et al., 2006) found that 1.2% of the sample met diagnostic criteria for HT with increased prevalence in children with Turner’s syndrome, celiac disease, and diabetes mellitus. Prevalence data based on gender in children were not reported; however, in adults the prevalence is 3.5 per 1,000 women and 0.8 per 1,000 men, with women being 10 to 15 times more likely to present with the disorder. The medical picture of HT is in line with other forms of hypothyroidism, already noted above.
Despite the fact that HT is the most common cause of acquired hypothyroidism, there is surprisingly little data on cognitive functioning. There have, however, been studies conducted that associate HT with severe mental health-related issues. For example, in his review of the clinical profile of individuals with HT, Odeke and Nagelberg (2006) described the possibility of psychosis, depression, dementia, memory loss and lack of energy as complicating features. Detlef and colleagues (2001) found that 6.1% of a large sample of inpatients with various diagnoses, including major depression and psychotic disorders, exhibited some type of thyroid dysfunction. Less than 1% of their sample met criteria for HT, but of the 20 patients who did, 11 were diagnosed with major depression, 5 with bipolar disorder, 3 with schizoaffective disorder, and 1 with schizophrenia. Several words of caution are required regarding these findings. First, this was a correlational study and the fact that many of the psychiatric patients presented with thyroid dysfunction does not imply a causal relationship. Second, the research was done using psychiatric inpatients whose conditions are more severe than the typical mental health patient, let alone someone whose symptoms are due to thyroid dysfunction. Lastly, caution should be used when attempting to generalize from adult-based findings to children.
The research on HT with children has focused on a condition referred to as Hashimoto’s encephalopathy. Encephalopathy is a complication of HT that is a function of neurological deterioration either acutely or insidiously and is generally treatable and at least partially reversible with the use of steroids. It can manifest as a stroke-like event, with acute seizures, confusion or a decline in cognitive functioning (Chong, Rowland, & Utiger, 2003; Watemberg, Willis, & Pellock, 2000). The condition is typically found in adults, but has been reported in children. A single case report was provided by Watemberg and colleagues (2000) who described a 9-year-old girl who was brought to an emergency room with generalized tonic-clonic seizures that were followed by confusion and agitation. She was followed for approximately 6 months and demonstrated a pattern of relapse and partial recovery (she did not return to premorbid baseline in terms of maturity level or academics). These findings are in line with other case reports of both children and adults that were reviewed by Watemberg and colleagues (2000), but further research is necessary in this area.
More extreme deficiency or even absence of thyroid hormones results in a condition known as cretinism. The major symptoms of cretinism are failure of skeletal growth and maturation, and marked retardation in the development of intellect (Hadley & Levine, 2007). Review of the literature on this extreme type of hypothyroidism reveals that even in endemic areas where nutrition and healthcare are less developed, the rates are fairly low. Jalil, Mia, and Ali (1997), conducted an epidemiologic study of the prevalence rates of cretinism in individuals ranging in age from 2 to 45 years in two areas of , one that was hyperendemic and one that was not endemic for cretinism. The prevalence rate for cretinism in the hyperendemic area was 0.6%, while there were no cases of cretinism in the non-endemic area. Actual prevalence rates in the are unavailable through a literature search but the condition is most commonly found in those regions of the world where iodine deficiency is a problem. This is not the case in the , as there are many ways of obtaining the necessary levels of iodine, including from iodized salt.
Summary
The impact of hypothyroidism on the human body is multifarious, but continued work and collaboration between the medical and mental health fields will provide a clearer and more complete picture, particularly in children. In terms of neurocognitive functioning, those working with children who have either congenital or acquired hypothyroid conditions need to attend to the possibility of decreased performance on IQ testing, deficits in motor skills, problems encoding verbal information, and attention problems, including maintaining focus, inhibiting responses, and mental shifting. Mental health issues also are problematic for this group of patients in terms of depressive symptoms, anxiety, and in the case of encephalopathy associated with HT, confusion, agitation, memory deficits, and seizures. It also is important to keep in mind that the types of deficits that arise depend on the time during which the hypothyroid condition came to the fore and that the more severe the condition, the more extensive the deficits. Furthermore, it is necessary to keep in mind that not all children with hypothyroidism will manifest all of these deficits and there is yet to be a consensus about the types of deficits.
Hyperthyroidism
Hyperthyroidism occurs when excessive thyroid hormones are produced by the thyroid gland. Recall that thyroid hormones affect mitochondrial oxidative capacity, protein synthesis and degradation, tissue sensitivity to neurotransmitters, muscle fiber growth and development, and capillary growth (Goetz, 2003). The clinical manifestations of thyroid overactivity include: weight loss despite adequate appetite; insomnia; fatigue; palpitations; heat intolerance and sweating; diarrhea; deterioration of fine motor skills, particularly handwriting; muscle weakness; and eye symptoms (Fenton, Gold, & Sadeghi-Nejad, 2006).
Hyperthyroidism in children is a relatively rare occurrence and 95% of childhood cases of hyperthyroidism are the results of Graves’ disease. As such, the prevalence rate of Graves’ disease, which is 0.02% in childhood, approximates the general frequency of hyperthyroidism in children, but only accounts for 5% of the total cases of Graves’ disease in the general population (Fenton et al., 2006). The prognosis for children with Graves’ disease is generally quite good but can be considerably worse when it occurs in very young children or during the neonatal period. Children who experience the disease process at a much younger stage are more prone to prematurity, airway obstruction, and heart failure, which increases their mortality rate to as high as 16% (Fenton et al.). The peak age of incidence is between 10 and 15 years, with a prevalence of between 3 and 6 females for every male with the disease.
Based on the fact that the vast majority of cases of childhood hyperthyroidism are due to Graves’ disease, the majority of the research has been conducted on this specific disorder and the following discussion will therefore focus on the neurocognitive and psychological components of Graves’ disease. As stated above, Graves’ disease is the most common cause of hyperthyroidism in children. It is an immune-mediated disorder that results from the production of thyroid stimulating immunoglobulins (TSI) by stimulated B lymphocytes. TSI binds to the thyroid stimulating hormone (TSH) receptor to mimic the action of TSH, thereby stimulating thyroid growth and hormone overproduction (Ferry & Levitsky, 2006; Goetz, 2003).
The research on Graves’ disease in children is quite limited and therefore a significant portion of the data presented below is based on studies with adults. Segni and colleagues (1999) found that children with hyperthyroidism were more likely to exhibit behavioral disturbances including decreased attention span, difficulties with concentration, hyperactivity, sleep problems, tachycardia (i.e., cardiac arrhythmia involving rapid heart rate), tremor, and weight loss, the latter presenting despite appropriate appetite. Several studies of adults with Graves’ disease have garnered evidence of frontal lobe involvement as characterized by executive dysfunction. Tremont, Somerville, and Stern (2000) reported results using the Rey-Osterrieth Complex Figure Test (RCFT; Meyers & Meyers, 1995), finding that those adults with Graves ’ hyperthyroidism had poor performance relative to euthymic controls. Tremont and colleagues reported the results of single photon emission computed tomography (SPECT) on three female patients with Graves’ disease. For two of the patients, the investigators found hypoperfusion (i.e., less blood flow and, hence, lower glucose metabolism) that was prominent in the frontal lobes and the anterior temporal regions, while findings for the third patient were in the normal range. Both of the patients with abnormal SPECT findings demonstrated deficits on neuropsychological measures of verbal learning, figural fluency, and visual construction. The patient with normal SPECT findings did not reveal such deficits. Fukui, Hasegawa, and Takenaka (2001) reported the SPECT findings for a 67-year-old man who experienced progressive impairment of attention, memory, construction skills, and behavior with concomitant hand tremor and weight loss over a two year period. SPECT findings pointed to hypoperfusion bilaterally in the temperoparietal regions. Subsequent treatment with methimazole resulted in improved memory and construction abilities.
Stern, Robinson, Thorner, et al. (1996) surveyed the neuropsychiatric complaints of adults with Graves’ disease. Respondents reported significant issues with memory, attention, planning, and productivity. Trzepacz and colleagues (1988) examined the psychiatric and neuropsychological sequelae of patients with untreated Graves’ disease. Although surveying only a small number of participants (13 adults), a majority met criteria for major depressive disorder and generalized anxiety disorder relative to other hospitalized medical patients. On neuropsychological testing, the participants were found to have mild deficits in attention, memory, and complex problem solving.
Not all studies have found the presence of neuropsychological deficits. Vogel, Elberling, Hording and colleagues (2007) evaluated a sample of 31 consecutively referred patients, newly diagnosed with Graves’ disease. They found higher ratings in psychiatric measures prior to treatment but no differences on neuropsychological test performance. The authors further reported that thyroid levels did not correlate with neuropsychological test findings or psychiatric ratings. After reaching a euthyroid level, previously reported psychiatric and cognitive impairments decreased significantly.
Summary
Graves’ disease is the most common cause of hyperthyroidism in children, accounting for 95% of cases, but the condition is generally rare. As such, it is not surprising that research is scant on this age group. The studies reviewed indicated that both timing and severity of the disorder impact how significant the clinical manifestation will be. Prognosis for hyperthyroidism, including Graves’ disease, is generally quite good in children but when it occurs at a very young age or during the neonatal period, severe complications such as airway obstruction and congestive heart failure can occur and these conditions significantly increase mortality rates.
The primary deficits exhibited by individuals with Graves’ disease on neuropsychological measures indicate the presence of executive dysfunction. Specifically, Graves’ disease appears to be associated with greater impairment in attention, including the ability to sustain attention and inhibit behaviors. Problems with memory also have been noted based both on the administration of memory tests and self-reports of impairment. This suggests that individuals with Graves’ disease are likely to require more time and effort in order to learn and consolidate new information. Planning and organizational skills also are affected in these individuals, which can result in a work product that is less well developed than should be the case. Unlike hypothyroidism, decrements in intellectual functioning were not reported in individuals with hyperthyroid conditions. On the other hand, the psychological and neuropsychiatric sequelae do overlap with hypothyroidism, including the presence of high levels of depressive features and anxiety.
Disorders of the Adrenal Glands
The adrenal glands are located adjacent to the upper surface of each kidney and typically weigh between 3.5 and 4.5 grams (Hadley & Levine, 2007). In many mammals, the adrenal glands are composed of steroidogenic and chromaffin tissue. Steroidogenic tissue, as the name may imply, is the primary site where glucocorticoids, such as cortisol, are produced. The secretion of cortisol is effected by stimulation of the hypothalamic-pituitary-adrenal axis (HPA), whereby the hypothalamus secretes cortisol releasing hormone (CRH), which, in turn, stimulates the pituitary, resulting in the release of adrenocorticotropic hormone (ACTH). ACTH then works to cause the release of cortisol from the adrenal glands. The level of cortisol serves as part of a negative feedback loop, signaling the hypothalamic-pituitary component of the HPA to cease production of stimulating hormones (Blumenfeld, 2002). Chromaffin tissue is the area where mineralocorticoids, such as aldosterone, are produced. The steroidogenic tissue forms a cortical mass around the chromaffin tissue and is therefore often referred to as the adrenal cortex, while the chromaffin tissue is referred to as the adrenal medulla (Hadley & Levine, 2007).
As with the thyroid, adrenal dysfunction can result in either hypo- or hyperadrenalism.
Hypoadrenalism
Hypoadrenalism is also known as adrenal insufficiency (AI). In its classic form, AI is caused by Addison’s disease, which was first described in the 19th century by British physician Thomas Addison. In its primary form, Addison’s disease results from bilateral destruction of the adrenal glands. In its secondary form, adrenal insufficiency results from hypothalamic or pituitary dysfunction (Goetz, 2003). In either case, adrenal insufficiency is characterized by pigmentation of the skin and mucous membranes, nausea, vomiting, weight loss, muscle weakness, lethargy, and a tendency to faint (Victor & Ropper, 2001). In children, hypoglycemia also is present (Wilson & Speiser, 2006).
Primary adrenal insufficiency is rare. A more likely cause is iatrogenic, central adrenal insufficiency, but the exact prevalence is unknown. Adrenal insufficiency secondary to congenital adrenal hyperplasia occurs in 1 per 16,000 infants. Autoimmune adrenal insufficiency is more common in females than males and in adults than children. Adrenal insufficiency due to adrenoleukodystrophy occurs almost exclusively in males due to the X-linked inheritance, while secondary insufficiency is equally prevalent in males and females (Wilson & Speiser, 2006).
According to the American Academy of Pediatrics, Section on Endocrinology and Committee on Genetics (2000), cognitive functioning in children with disorders of hyposecretion of adrenal hormones has not been well studied, particularly in terms of neuropsychological functioning. Mercado, Wilson, Cheng, et al. (1995) provided a preliminary report on maternal accounts of children’s cognitive and behavioral functioning using a standardized questionnaire. They did not find significant differences in either behavioral or cognitive ratings for children at risk for congenital adrenal hyperplasia (CAH) who were either treated or not with prenatal dexamethasone. However, the authors did find what were referred to as “neurologically silent” white matter abnormalities and temporal lobe atrophy in both children and adults with CAH. Hirvikoski and colleagues (2007) evaluated a group of prenatally treated 7 to 17 year old children using an abbreviated version of the Wechsler Intelligence Scale for Children (Donders, 1997) and subtests of A Developmental Neuropsychological Assessment (NEPSY; Korkman, Kirk, & Kemp, 1998). Although trends toward lower verbal processing speed were found in children with CAH who were treated prenatally with dexamethasone, these differences did not hold up following statistical correction.
Chrousos (2004) reviewed the expected neurocognitive manifestations of Addison’s disease, listing the following: lack of energy (i.e., asthenia), depression, memory dysfunction, executive dysfunction, hyperalgesia (i.e., hypersensitivity to pain), fatigue, and poor sleep quality. The primary adrenal hormone involved is posited to be cortisol as it is involved in hippocampal function and also fluctuates across the sleep cycle (see Payne & Nadel, 2004 for a review of the literature and potential mechanisms of action). Blair, Granger, and Razza (2005) studied the relationship between cortisol reactivity and executive function in preschool children attending a Head Start program. They found that moderate elevation of cortisol, followed by a down regulation, was positively associated with executive functions such as cognitive flexibility and self-regulation. In rats that had their adrenal glands removed, the physiological response of the dentate gyrus of the hippocampus to stimulation was found to be attenuated (Hadley & Levine, 2007). These results lend indirect support to the relationship between hypofunction of the adrenal glands and deficits in memory and executive function.
Hyperadrenalism
The clinical components of Cushing’s disease were first described by American neurosurgeon, H. W. Cushing’s in 1932. The features included truncal obesity, reddish-purple cutaneous striae (i.e., irregular bands of skin that look like bands), dryness and pigmentation of the skin, fragility of skin blood vessels, excessive facial hair, baldness, cyanosis and mottling of the skin and extremities, osteoporosis, muscle weakness, and hypertension (Victor & Ropper, 2001). The cause of Cushing’s disease is a pituitary microadenoma (i.e., a tumor that is less than 1 cm in size), that produces ACTH, which, in turn, stimulates the adrenal glands. These tumors are often referred to as basophils because of their affinity for specific stains that give them a purplish or blue appearance. Basophils are further classified by the type of hormone they secrete. For example, the tumors involved in Cushing’s disease are corticotrophic because their cells secrete ACTH. When the combination of symptoms reported above is due to a primary adrenal tumor, ectopic production of ACTH by other tumors (e.g., lung carcinoma), or prolonged administration of glucocorticoids (e.g., prednisone), the term Cushing’s syndrome is appropriate (Adler & Dipp, 2006; Victor & Ropper, 2001).
The majority of cases of Cushing’s syndrome are due to exogenous glucocorticoids such as would be used in the treatment of inflammation or infection. The annual incidence of endogenous Cushing’s syndrome has been estimated at 13 cases per million individuals, of which approximately 70% are due to Cushing’s disease, 15% to ectopic ACTH secretion, and 15% to primary adrenal tumor. The female-to-male ratio is 5:1 and the peak incidence of Cushing’s syndrome occurs in persons aged 25 to 40 years. Ectopic ACTH secretion occurs later in life usually due to lung cancer (Adler & Dipp, 2006).
The impact of excess glucocorticoid exposure on the brain is quite significant, particularly in light of the fact that the brain continues to develop throughout childhood and adolescence. Those areas of the brain most sensitive to the impact of corticosteroids include the hippocampus, prefrontal cortex, and amygdala (Romeo & McEwen, 2006). In their review of the animal literature, Romeo and McEwen highlighted the impact of corticosterone, which is the animal analogue of cortisol, on the rat brain including the hippocampus, prefrontal cortex, and amygdala. The hippocampus is a brain region that is critical for learning and memory consolidation (Mesulam, 2000). Prolonged exposure to corticosterone resulted in a reduction of the branching of the apical dendrites of the CA3 region of the hippocampus, with the outcome being spatial memory impairment. These results were reversed when the animals were placed in a relatively stress-free environment (which included reduction of the level and time of exposure to corticosterone) or when antidepressant medication was administered (Romeo & McEwen, 2006).
The prefrontal cortex (PFC) is involved in executive functioning, emotion regulation and fear extinction (Sotres-Bayon, Cain & LeDoux, 2006). Romeo and McEwen (2006) reported that in adult rats, stress due to chronic restraint (i.e., six hours per day for a period of 3 weeks) resulted in reductions in both apical dendritic branching and spine density of the medial prefrontal cortical pyramidal neurons including the areas of anterior cingulate cortex and pre-limbic area. In contrast to the medial PFC, orbitofrontal cortex showed growth as a result of repeated stress. PFC atrophy due to stress-induced remodeling is associated with impairment of attention set-shifting and inhibiting impulses that are not appropriate to the context (Liston et al., 2006; Mesulam, 2000).
In contrast to the impact of stress on the morphology of the hippocampus and PFC, adult rats showed dendritic hypertrophy in the basolateral, but not the central nucleus of the amygdala. These animals exhibited what were referred to as elevated anxiety-like behaviors that remained even after three weeks of stress-free recovery (Vyas, Pillai, & Chattarji, 2004).
In line with the animal model described above, Chrousos (2004) reported the following neurocognitive findings in his review of the clinical sequelae of Cushing’s syndrome and glucocorticoid hypersensitivity: anxiety, insomnia, depression, memory dysfunction and executive dysfunction. Furthermore Arnaldi and colleagues (2003), reported that between 50 and 80% of patients with Cushing’s syndrome met diagnostic criteria for major depression. Moreover, children with hypersecretion of cortisol were reported to exhibit an elevated level of obsessive compulsive features. In a study of 48 adults diagnosed with Cushing’s disease, Starkman and colleagues (2001) found that their patient group had statistically significant lower verbal IQ scores relative to non-patient controls. What is more, verbal learning and memory were significantly lower in the patient group, including delayed recall of verbal material. In contrast to the deficits noted on the verbal components of memory, memory for visual material was not significantly different between the patients and control participants. Patients with Cushing’s disease also were significantly more depressed than control participants. Arnaldi and colleagues (2003) further reported that many of the psychological features associated with Cushing’s syndrome remitted after normalization of cortisol levels, typically with surgery involving removal of the adrenal glands. However, there is data suggesting that problems with social, psychological, and cognitive functioning continue to plague many patients despite normal cortisol levels. At the time of diagnosis and one year after normalization of cortisol levels, Merke and colleagues (2005) performed clinical, cognitive, and psychological testing, as well as magnetic resonance brain imaging (MRI) on 11 children with Cushing’s syndrome ranging in age from 8 to 16 years. The authors reported that there were no significant differences in IQ between the two groups and no psychopathology was identified at baseline. On the other hand, baseline neuroimaging indicated that children with Cushing’s syndrome had significantly smaller cerebral volumes, enlarged ventricles, and smaller amygdala. Hippocampal volume also was smaller in the patient group, but this difference did not reach statistical significance. The post-treatment evaluation revealed significant reversal of cerebral atrophy marked by an increase in total cerebral volume and decrease in ventricular volume. Despite the physiological reversal of atrophy, the children with Cushing’s syndrome experienced a significant decline in Full Scale IQ, falling from a standard score of 112 (+/-19 points) to 98 (+/-14 points) at one year. Also noted was a decline in school performance without any associated psychopathology. The reasons for the decline in performance is not clear, but the authors posit the possible impact of prior cortisol excess, the impact of surgery, and a relative cortisol deficiency subsequent to treatment. In terms of IQ scores, one also needs to consider regression towards the mean as a potential factor as the children in this group scored in the high average range relative to normative data (Healy & Goldstein, 1978).
Mental Health and the Hypothalamic-Pituitary-Adrenal Axis (HPA)
The importance of the HPA in our mental health is becoming ever clearer. The purpose of this section is to provide a brief summary of the research on the role of the HPA on a variety of disorders that are frequently encountered by mental health professionals. Lopez and colleagues (1997) reviewed the regulatory role of serotonin receptors by the HPA, specifically in terms of its implications for the neurobiology of suicide. The primary hypothesis is that 5-HT receptor changes that have been observed in individuals who committed suicide may be worsened by or even the result of HPA overactivity. In support of their hypothesis, Lopez and colleagues cited a number of lines of research. First, in terms of animal models, they reported the well-known finding that chronic unpredictable stress produces high levels of corticosteroid production. Second, chronic stress results in specific changes to 5-HT receptor sites with general increases in cortical receptor sites, but decreases in hippocampal regions. Moreover, chronic administration of antidepressant medication prevents many of the changes noted in 5-HT receptors that have been observed to result from stress and the medication also reverses overactivity of the HPA. While not conclusive in and of itself, this review highlights the potential role of HPA in depression and suicidal behavior.